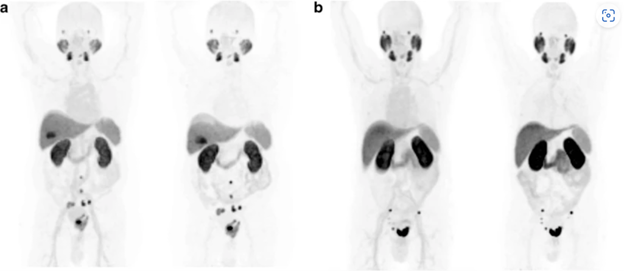
Image adapted from Giesel, F.L., Hadaschik, B., Cardinale, J. et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging 44, 678–688 (2017). https://doi.org/10.1007/s00259-016-3573-4. Image has not been altered in any way. Link here for Creative commons license to publish.
CPDC holds exclusive licensing rights for PSMA-1007 from ABX GmbH (Radeberg, Germany) to manufacture, commercialize and distribute the imaging agent in Canada. CPDC is focused on bringing a critically important diagnostic tool for prostate cancer to the Canadian market.